
Carbendazim and hydrochloric acid structure and physical parameters.

diagram 7: Structure of acid surface oxides. [Garten and Weiss/Mattson and

Crystal structure of hydrogen chloride

glacial acetic acid, sodium hydroxide or hydrochloric acid (to adjust
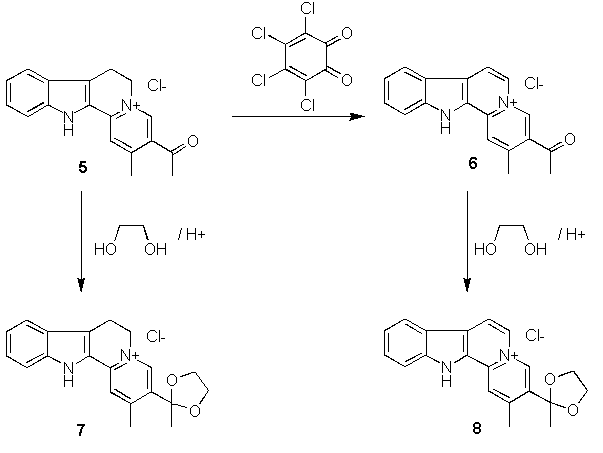
and treatment with ethyleneglycol and aqueous hydrochloric acid).

Inactive: Sodium Hydroxide and/or Hydrochloric Acid (neutralized to adjust
Hydrochloric acid is a strong acid.

structure of HCL

(ii) Amino Acid Structure and Chemical and Physical Characteristics

Carbon dioxide, steam, sulfur dioxide, hydrogen sulfide, hydrochloric acid,

The main uses of the hydrochloric acid are mention.. Amorphous form:
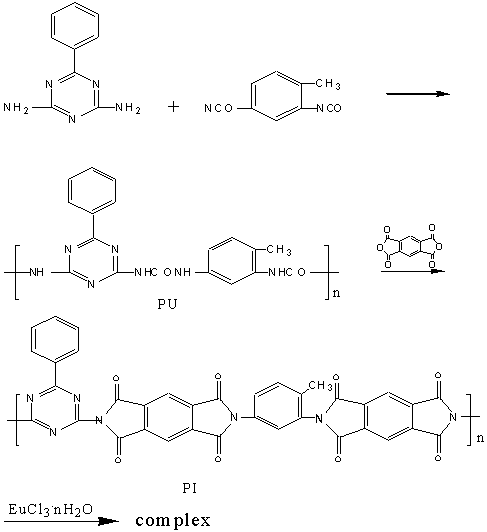
Europium oxide was dissolved in dilute hydrochloric acid (6mol/L),

Water and hydrochloric acid

base of arbidol (V), which is treated with aqueous hydrochloric acid .

converted to lobucavir by refluxing with aqueous hydrochloric acid (1).
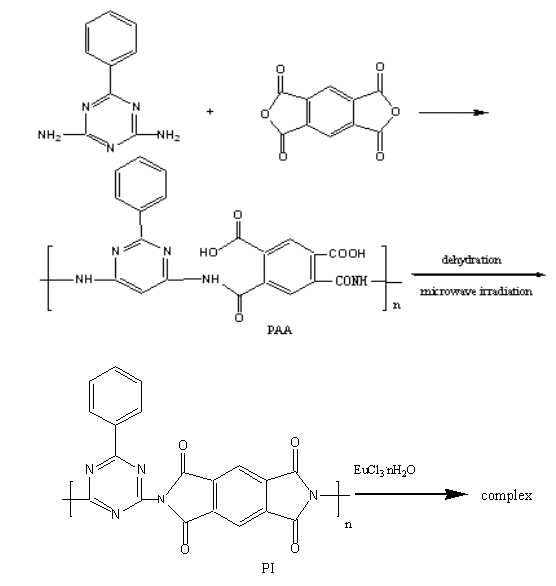
Europium oxide was dissolved in hydrochloric acid (6mol/L), and the solution

CORROSION INHIBITION OF COPPER IN 0.5 M HYDROCHLORIC ACID BY

formed by the interaction of sodium molybdate with hydrochloric acid.
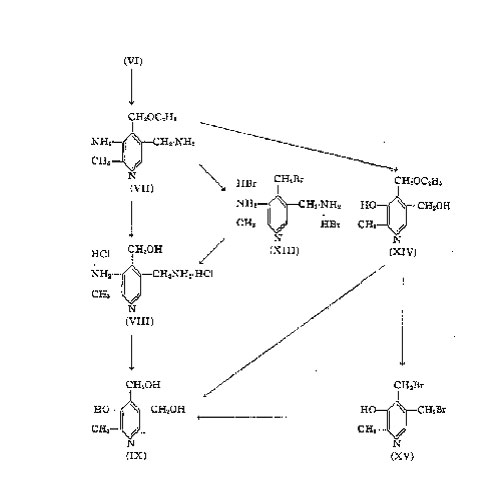
The latter compound upon treatment with tin and hydrochloric acid yields the

were prepared by progressive additions of hydrochloric acid



